ARTICLE
It is pleasing to see that finally, the FDA in the USA, has recognised that people with silicone breast implants are prone to an illness known as Breast Implant Associated Illness as well as the disease known as ALCL.
At Avenue Aesthetic Surgery we encounter many patients who have undiagnosed (silent) rupture of their silicone breast implants. Many of these patients have significant symptoms.
Below is part of the recent memorandum published by the FDA.
“Over the past several years, the FDA has received new information pertaining to risks associated with breast implants, including breast implant-associated anaplastic large cell lymphoma (BIA-ALCL) and systemic symptoms commonly referred to as breast implant illness (BII) that some patients attribute to their implants, which can include fatigue, brain fog, muscle or joint pain and rash.
The FDA has taken a number of steps to better understand and address risks associated with breast implants, including convening the General and Plastic Surgery Devices Advisory Panel in 2019 to discuss the long-term benefits and risks of breast implants indicated for breast augmentation and reconstruction. The meeting covered a range of important topics on breast implant safety, including characterization of BIA-ALCL incidence and risk factors, and methods for assessing systemic symptoms. The Panel gave recommendations on these topics, including recommending that FDA require a boxed warning in breast implant labelling and a standardized checklist as part of the informed consent process, revise the MRI screening recommendations for silent ruptures of silicone gel-filled breast implants and provide greater transparency regarding materials present in breast implants. The Panel also discussed the role of the patient device card in providing important information about the patient’s breast implant.”
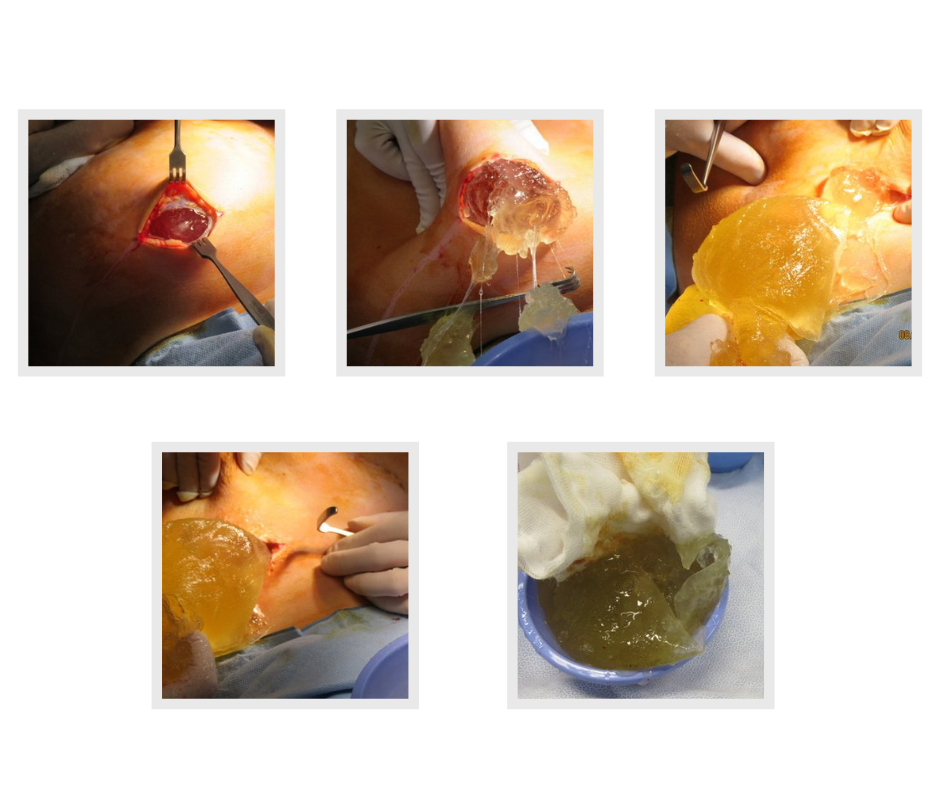
One of our first cases this week at Avenue Aesthetic Surgery post COVID 2.0 restrictions was removing this leaking Silimed textured 625cc high cohesive gel breast implant from behind the pectoral muscle. The woman has severe symptoms of Breast Implant Illness including memory loss, skin rashes, joint pains and fatigue. A reconstruction was performed using fat transfer from her hips and thighs. She was unaware that the implant had ruptured and the other implant was intact.
ARTICLE
FDA Acknowledges Breast Implant Illness
It is pleasing to see that finally, the FDA in the USA, has recognised that people with silicone breast implants are prone to an illness known as Breast Implant Associated Illness as well as the disease known as ALCL.
At Avenue Aesthetic Surgery we encounter many patients who have undiagnosed (silent) rupture of their silicone breast implants. Many of these patients have significant symptoms.
Below is part of the recent memorandum published by the FDA.
“Over the past several years, the FDA has received new information pertaining to risks associated with breast implants, including breast implant-associated anaplastic large cell lymphoma (BIA-ALCL) and systemic symptoms commonly referred to as breast implant illness (BII) that some patients attribute to their implants, which can include fatigue, brain fog, muscle or joint pain and rash.
The FDA has taken a number of steps to better understand and address risks associated with breast implants, including convening the General and Plastic Surgery Devices Advisory Panel in 2019 to discuss the long-term benefits and risks of breast implants indicated for breast augmentation and reconstruction. The meeting covered a range of important topics on breast implant safety, including characterization of BIA-ALCL incidence and risk factors, and methods for assessing systemic symptoms. The Panel gave recommendations on these topics, including recommending that FDA require a boxed warning in breast implant labelling and a standardized checklist as part of the informed consent process, revise the MRI screening recommendations for silent ruptures of silicone gel-filled breast implants and provide greater transparency regarding materials present in breast implants. The Panel also discussed the role of the patient device card in providing important information about the patient’s breast implant.”
One of our first cases this week at Avenue Aesthetic Surgery post COVID 2.0 restrictions was removing this leaking Silimed textured 625cc high cohesive gel breast implant from behind the pectoral muscle. The woman has severe symptoms of Breast Implant Illness including memory loss, skin rashes, joint pains and fatigue. A reconstruction was performed using fat transfer from her hips and thighs. She was unaware that the implant had ruptured and the other implant was intact.
